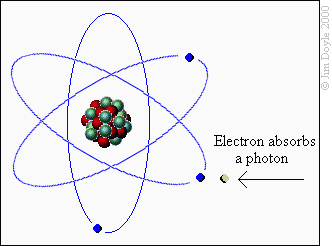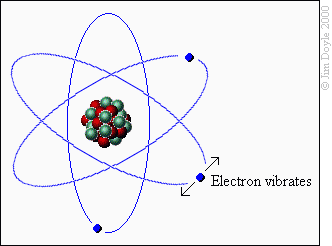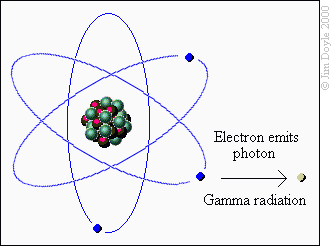
This will be a brief but informative overview of how fireworks are made. Fireworks are made of chemicals. When an external stimulus like heat is applied, the chemical bonds of the chemicals are broken and excess energy is released.
When certain chemicals are “excited” their electrons are released as “photons”. Photons are particles of light. Photons vibrate at specific frequencies or wavelengths and those wavelengths translate to colors.
Common chemicals used in fireworks are as follows:
Oxidizers – These chemicals provide the oxygen for the fire to burn. Since fireworks carry their own oxygen, they can burn hotter and faster than fires that need to rely on oxygen from the atmosphere. Some common fireworks oxidizers are Potassium Nitrate (KNO3) and Potassium Perchlorate (KCLO4).
Fuels – These chemicals provide the energy to create the heat need to break apart the chemical bonds. Some common low temperature fuels are Charcoal and Sulfur. High temperature fuels are metal powders such as Aluminum, Magnesium or alloys of both such as Magnalium (MG/AL). Recently it has been discovered that natural rubbers, like Parlon help act as both a fuel and also a Chlorine Donor. The Chlorine atom helps to make the colors of fireworks richer and more beautiful.
Color Salts – Finally the chemicals that actually produce the colors when heated are called color salts. For fireworks the following chemicals are most common:
- Red – Strontium
- Green – Barium
- Blue – Copper
- Yellow – Sodium
- White – Titanium
Colors like Purple can be achieved by mixing the chemicals needed to produce both red and blue. Orange would be achieved with a mixture of red and green and so forth.
 Blue is one of the most difficult fireworks colors to produce because the energy that Copper is excited at and gives off a photon is comparatively low. Therefore, in the heat of a firework, the temperature is often too hot and instead of pure blue light being produced, white light is produced. Therefore, many blue formulas use special fuels that burn at low temperatures in order to allow the blue color to be seen.
Blue is one of the most difficult fireworks colors to produce because the energy that Copper is excited at and gives off a photon is comparatively low. Therefore, in the heat of a firework, the temperature is often too hot and instead of pure blue light being produced, white light is produced. Therefore, many blue formulas use special fuels that burn at low temperatures in order to allow the blue color to be seen.
You can make your own Blue flames with a product called Campfire Blue. This product contians copper salts that when sprinkled on a fire like a campfire or fireplace, the flames will burn blue.
You can purchase it with no minimum order and low shipping cost here:
http://www.usfireworks.biz/items/dm819-campfireblue.htm
Fireworks can inspire a whole world of learning. From chemistry, to physics, to artistic choreography and computer firing control. The modern Pyrotechnition is truly a well versed expert in many fields.
Check back often for more articles on the art, science and technique of fireworks!